Acids Worksheets Results
Intro to Acids & Bases Worksheet
The Arrhenius definition of acids and bases is one of the oldest. An Arrhenius acid is a substance that when added to water increases the concentration of H+ ions present. The chemical formulas of Arrhenius acids are written with the acidic hydrogens first. An Arrhenius base is a substance that when added to water increases the concentration of ...
https://url.theworksheets.com/3gh280 Downloads
Preview and Download !


Worksheet 1 Acids, Bases & Indicators
KISS Resources for NSW Syllabuses & Australian Curriculum page 1 Usage & copying is permitted according to the SITE LICENCE CONDITIONS only Chem Mod.6 “Acid-Base Reactions” Worksheets
https://url.theworksheets.com/2whv196 Downloads
Preview and Download !


MATTER AND MATERIALS Acids and Bases
REACTIONS OF ACIDS AND BASES SECTION A QUESTION 1 Read the following extract and answer questions 1.1-1.4. A science learner placed a strip of blue litmus paper and a strip of pink litmus paper in a glass dish. She then dripped a few drops of dilute sulphuric acid on each strip
https://url.theworksheets.com/2sai98 Downloads
Preview and Download !


Acids, Bases and Salts - IGCSE STUDY BANK
www.igcse.at.ua www.igcse.at.ua Acids, Bases and Salts THE THEORY of ACIDS and ALKALIS and a few technical terms: o Acids are substances that form hydrogen ions (H+ (aq)) when dissolved in water eg hydrochloric acid HCl gives H+ (aq) and Cl-(aq) ions, sulphuric acid H 2 SO 4 gives 2H+ (aq) and SO
https://url.theworksheets.com/20or186 Downloads
Preview and Download !


Worksheet18 acidbase Key
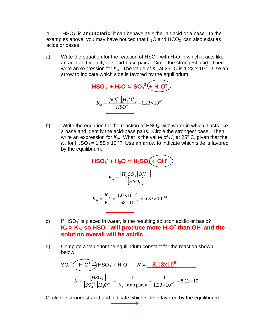
Worksheet 18 - Acids and Bases The Brønsted-Lowry definition of an acid is a substance capable of donating a proton (H+), and a base is a substance capable of accepting a proton.For example, the weak acid, HF, can be dissolved in water, giving the reaction: HF (aq) + H 2O (l) ' H 3O + (aq) + F-(aq) acid conjugate base
https://url.theworksheets.com/2e7w241 Downloads
Preview and Download !
Week 8 Worksheet: Chapter 10 Acids and Bases
Week 8 Worksheet: Chapter 10 Acids and Bases I. Identifying acid/base theories. For each molecule or ion in the table, identify whether it can act as an acid or a base and put a checkmark under each theory or theories that describe it. Molecule/Ion Acid or Base Arrhenius Bronsted-Lowry Lewis Br-base x CN-base x H2CO3 acid x x NH3 base x HNO2 ...
https://url.theworksheets.com/3dur184 Downloads
Preview and Download !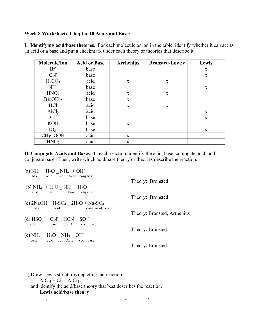

Worksheet Acids - STEM Learning
Acids & alkalis for KS3 Science - Worksheet (Answers) 1. Colour the pH scale and add the following labels: Neutral, Strong acid, weak acid, strong alkali and weak alkali.
https://url.theworksheets.com/1bxj552 Downloads
Preview and Download !


ACIDS AND BASES WORKSHEET NAME GRADE DATE acid
Acids are corrosive. What does this mean? • What is an indicator? • What is litmus paper? • What colors are they available in? • How do they change with acids and bases? Name one use for each of the following acids: Sulfuric Acid , Hydrochloric Acid, Nitric Acid, Citric Acid and Carbonic Acid. What is a base? How does it taste and feel?
https://url.theworksheets.com/27b880 Downloads
Preview and Download !
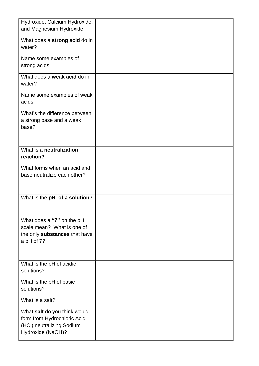

Acid and Base Worksheet
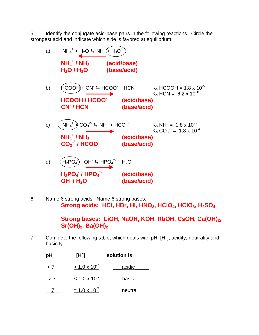
1) Using your knowledge of the Brønsted-Lowry theory of acids and bases, write equations for the following acid-base reactions and indicate each conjugate acid-base pair: a) HNO 3 + OH- b) CH 3NH 2 + H 2O c) OH-+ HPO 4-2 2) The compound NaOH is a base by all three of the theories we discussed in class.
https://url.theworksheets.com/3qau85 Downloads
Preview and Download !


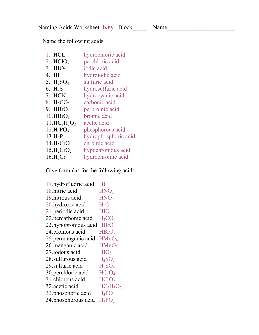
Naming Acids Worksheet Key Name the following acids
Naming Acids Worksheet Key Block_____ Name_____ Name the following acids 1. HCL hydrochloric acid 2. HClO4 perchloric acid 3. HIO3 iodic acid 4. HI hydroiodic acid 5. H2SO4 sulfuric acid 6. H2S hydrosulfuric acid 7. HCN hydrocyanic acid 8. H2CO3 carbonic acid 9.
https://url.theworksheets.com/3qat93 Downloads
Preview and Download !

Next results >>