Acids Worksheets Results
Worksheet-Organic Acids and Bases II
Worksheet – Organic Acids and Bases II Amino Acids Acids are proton donors in aqueous solutions: HA + H2O H3O + + A-acid base conjugate conjugate base acid The strength of an acid is measured by the [H3O +] at equilibrium. The higher the concentration, the stronger the acid.
https://url.theworksheets.com/1by076 Downloads
Preview and Download !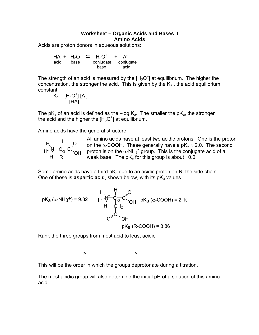
Section Name Date 5.1 Acids and Bases
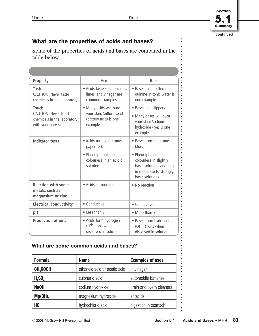
Acids have a pH below 7 and bases have a pH above 7. Neutral solutions have a pH of 7. On the pH scale, one unit of change represents a 10-fold change in the degree of acidity or basicity. For example, a two unit drop in pH is a 102 or 100 times increase in acidity.
https://url.theworksheets.com/1bxr115 Downloads
Preview and Download !

Acid and Base pH Calculations Supplemental Worksheet KEY
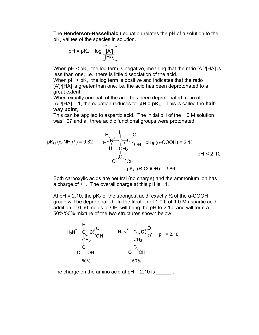
are the concentrations of H3O+ ions, OH-ions, weak acids and weak bases. So here we have [H3O+] = n/V = 0.01 moles / 0.3 L = 0.033 M. Note we had to convert back to concentrations from moles. The volume of the solution is 0.3 L because 200 mL was added to 100 mL. Step 3: What is the pH of the solution?
https://url.theworksheets.com/1bxg150 Downloads
Preview and Download !


REVIEW PROBLEMS ACIDS WORKSHEET 26 (PRACTICE)
REVIEW PROBLEMS ACIDS WORKSHEET 26 (PRACTICE) 1. Using the Ka values, calculate the percent dissociation in a 0.20 M solution of each of the following acids. a. nitric acid (HNO 3) b. nitrous acid (HNO 2) Ka = 4.1 x 10-4 c. phenol (HOC 6 H 5) Ka = 1.6 x 10-10 d. How is the percent dissociation related to the Ka value for the acid?
https://url.theworksheets.com/13ev149 Downloads
Preview and Download !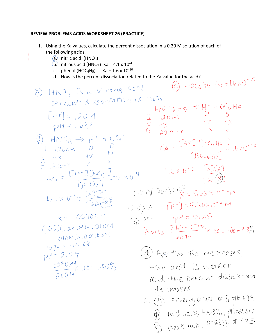

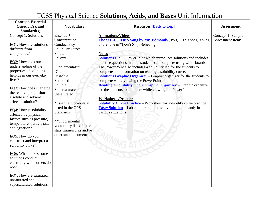
TCSS Physical Science Unit 5 Solutions, Acids, and Bases ...
The Strengths and Weaknesses of Acids and Bases (3:47) – Informational video by Georgia Zaidan and Charles Morton about Notes Acid and Base PowerPoint – PowerPoint for the notes of Acids and Bases Acids and Base Graphic Organizer – Graphic organizer for the students to use while viewing the PowerPoint Practice/Worksheets
https://url.theworksheets.com/2ojn106 Downloads
Preview and Download !

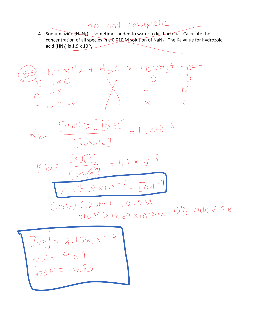
Worksheet 20 – Polyprotic Acids and Salt Solutions
Worksheet 20 – Polyprotic Acids and Salt Solutions K a Acid Base K b strong acid HNO 3, HI, HCl, etc NO 3-, I-, Cl-, etc negligible basicity 1.3 x 10-2 HSO 4-SO4 2-7.7 x 10-13 7.1 x 10-4 HNO 2 NO 2-1.4 x 10-11 6.8 x 10-4 HF F-1.5 x 10-11 1.8 x 10-5 CH 3COOH CH 3COO-5.6 x 10-104.5 x 10-7 H 2CO 3 HCO 3
https://url.theworksheets.com/3joz94 Downloads
Preview and Download !


Summary Sheet Year 8 Acids and Alkalis - Turton School
Summary Sheet Year 8 Acids and Alkalis: The pH Scale The pH of a solution depends on the strength of the acid: strong acids have lower pH values (pH 1-3) than weak acids. Neutral solutions have a pH of 7. For example, pure water. We can use indicators to test the pH of a substance. An indicator is a substance that
https://url.theworksheets.com/3qav511 Downloads
Preview and Download !


<< Previous results Next results >>