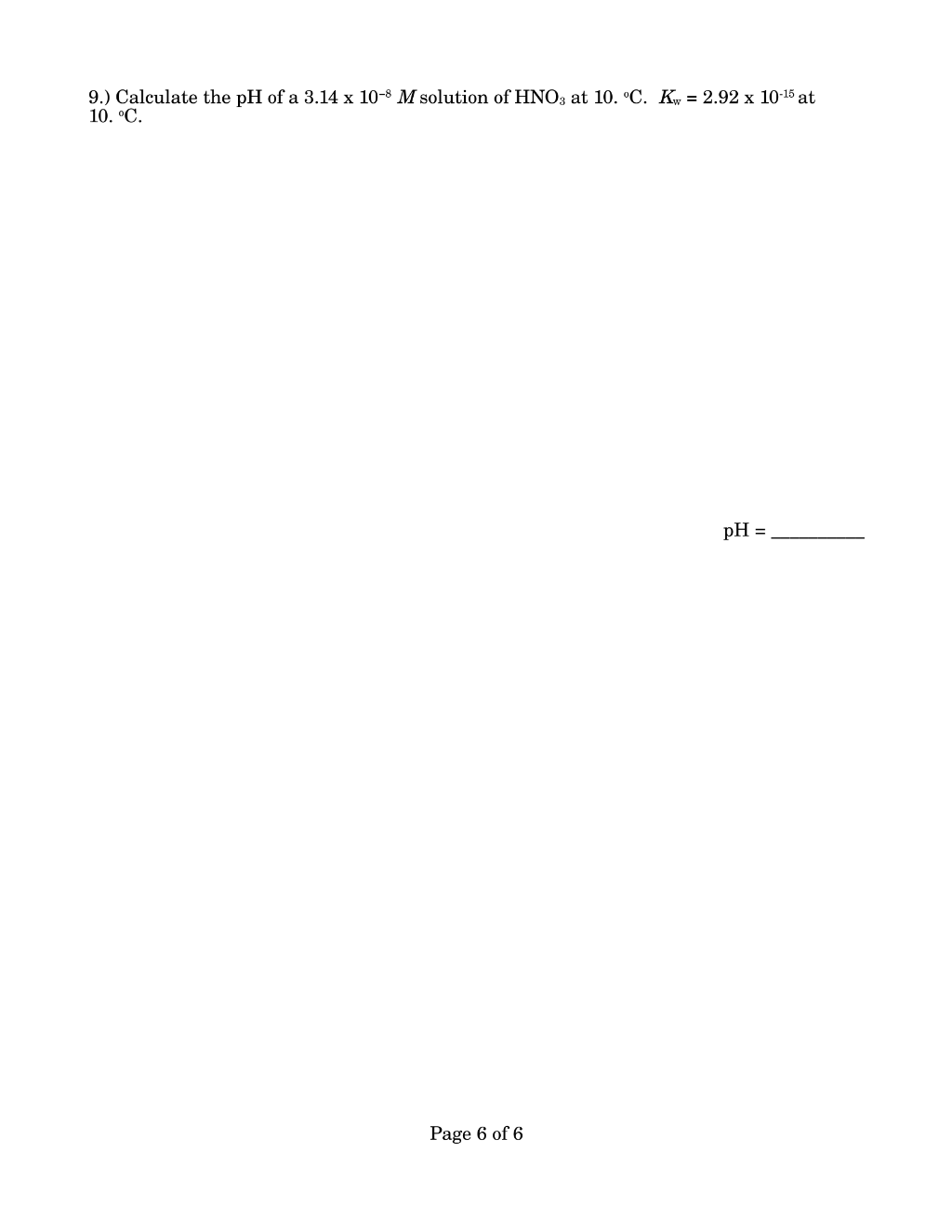
Preview 1

Ka’s are for acids and Kb’s are for bases. The relationship between Ka and Kb for a weak acid and it’s comjugate base is given by: For any weak acid, shown as HA, the percent dissociation is given by: 1.) Calculate the pH of the following solutions: 0.512 M HClO4 and 0.512 M HF (Ka = 6.76 x 10−4) pH HClO4 _____ pH HF_____ Page 1 of 6
Preview 1

Preview 2

Preview 3

Preview 4

Preview 5

Preview 6