Preview 1

Worksheet 18 - Acids and Bases The Brønsted-Lowry definition of an acid is a substance capable of donating a proton (H+), and a base is a substance capable of accepting a proton.For example, the weak acid, HF, can be dissolved in water, giving the reaction: HF (aq) + H 2O (l) ' H 3O + (aq) + F-(aq) acid conjugate base
Preview 1

Preview 2

Preview 3
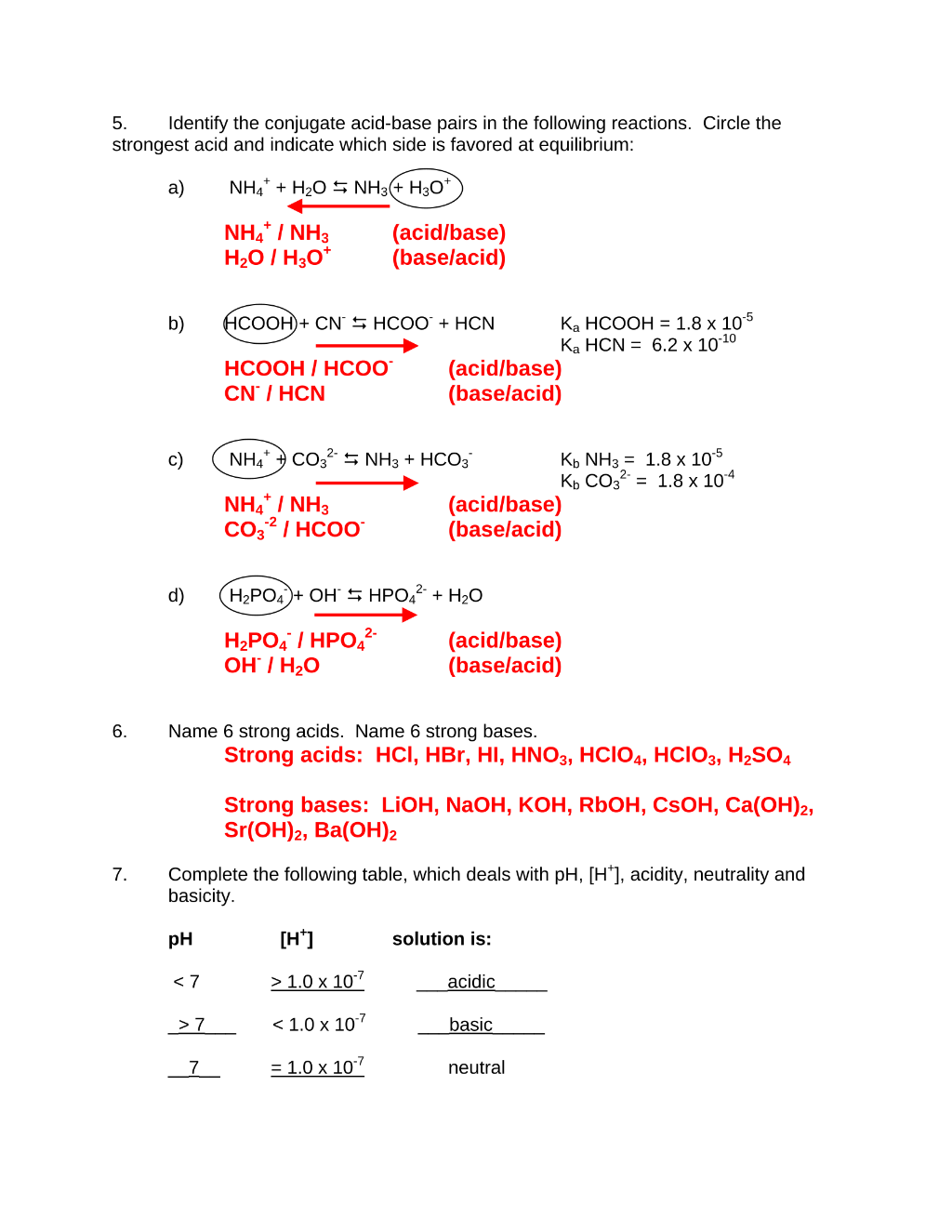
Preview 4
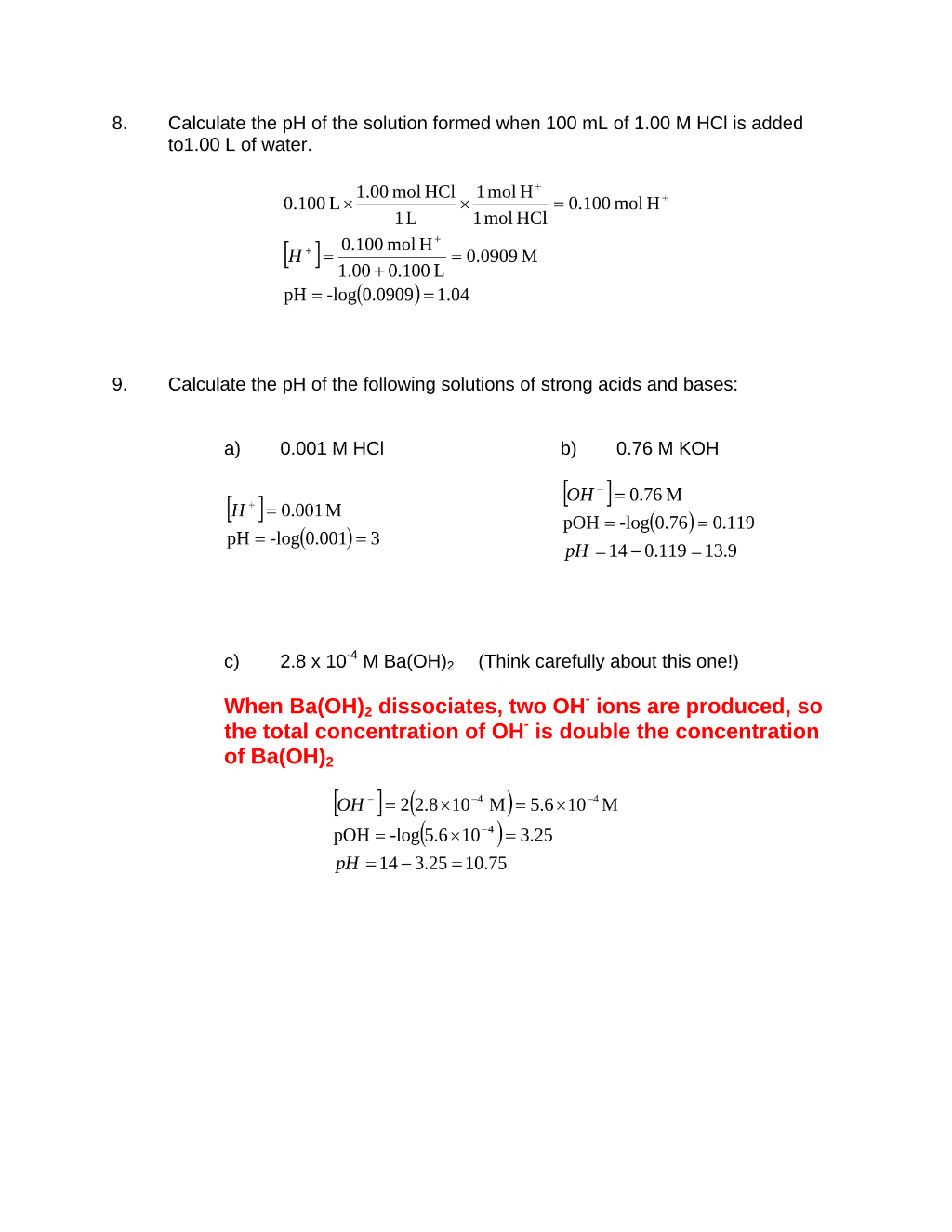
Preview 5

Preview 6
