Preview 1

Apply the Law of Conservation of Mass [a relation stating that in a chemical reaction, the mass of the products equals the mass of the reactants]. to get the same number of atoms of every element on each side of the equation. Tip: Start by balancing an element that appears in only one reactant and product.
Preview 1

Preview 2

Preview 3
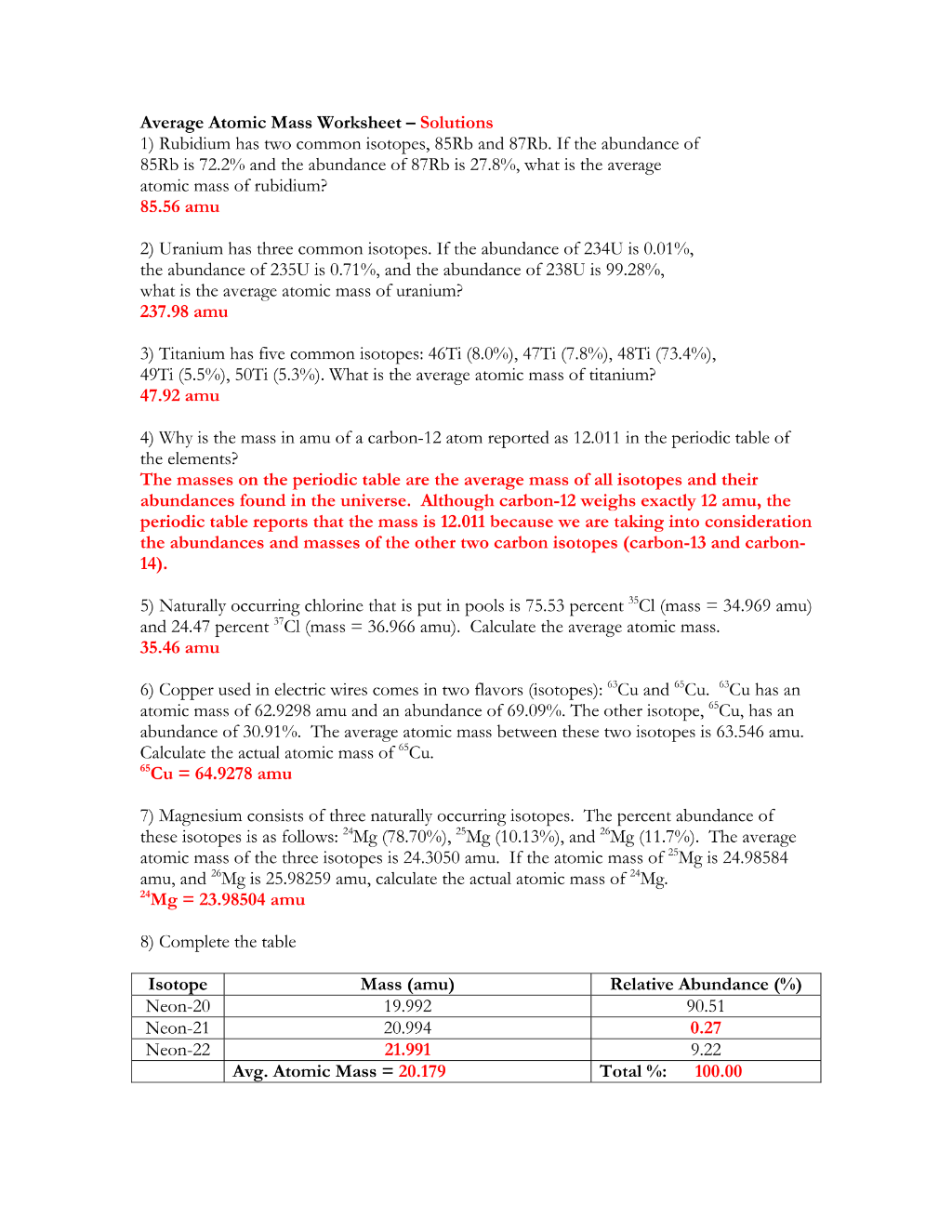
Preview 4

Preview 5

Preview 6
