Balancing Worksheets Results
Balancing Word Equations Chapter 9 - My Chemistry Class
Worksheet Balancing Word Equations Chapter 10 (Remember the following are diatomic: H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2) The coefficients should add up to the number at the end that is in parenthesis. 1. carbon + oxygen carbon dioxide (0) 2. copper + silver nitrate copper(II) nitrate + silver (4) 3.
https://url.theworksheets.com/19uu80 Downloads
Preview and Download !


Balancing REDOX Reactions: Learn and Practice
Balancing REDOX Reactions: Learn and Practice Reduction-Oxidation reactions (or REDOX reactions) occur when the chemical species involved in the reactions gain and lose electrons. Oxidation and reduction occur simultaneously in order to conserve charge. We can “see” these changes if we assign oxidation numbers to the reactants and products.
https://url.theworksheets.com/19v863 Downloads
Preview and Download !


Types of Reactions Worksheet
Types of Reactions Worksheet – Solutions Balance the following equations and indicate the type of reaction taking place: 1) 3 NaBr + 1 H3PO 4 1 Na 3PO 4 + 3 HBr Type of reaction: double displacement 2) 3 Ca(OH) 2 + 1 Al 2(SO 4)3 3 CaSO 4 + 2 Al(OH) 3 Type of reaction: double displacement 3) 3 Mg + 1 Fe 2O3 2 Fe + 3 MgO Type of reaction: single displacement 4) 1 C2H4 + 3 O2 2 CO 2 + 2 H2O
https://url.theworksheets.com/33q659 Downloads
Preview and Download !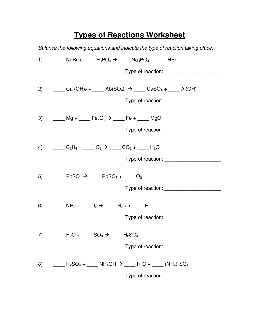


More Practice Balancing Redox - Livingston Public Schools
Balance everything except H and O by inspection. (If no ions are present, finish balancing the equation by inspection. Check to see that each element is balanced.) If ions are present, balance the charge by adding H + for acid solutions or OH - for basic solutions. Finish balancing the equation by adding 1-120.
https://url.theworksheets.com/36bv110 Downloads
Preview and Download !


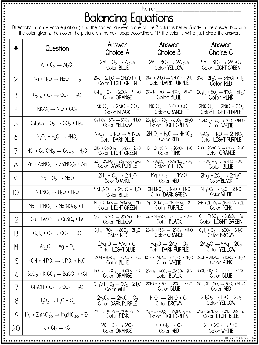
Balancing Equations
Directions: Balance each equation & find the correct answer in one of the 3 columns below. Shade in the answer box with the color given & then color the picture on the next page accordingly.
https://url.theworksheets.com/19vl76 Downloads
Preview and Download !

<< Previous results Next results >>