Periodic Table Worksheets Results
the-periodic-table-worksheet
The Periodic Table Directions: Use Chapter 4 Section 2 and the Periodic Foldable to complete this worksheet. I. List the atomic numbers Of the elements in Period 2. 2. List the symbols of the elements in Period 2. 3. Name the elements in Period 2 that are metals. 4. Name the elements in 2 that are nonmetals. 5. Which element in period 2 has the ...
https://url.theworksheets.com/4heq168 Downloads
Preview and Download !


Chemistry: The Periodic Table and Periodicity - Mrs. Klatt's Science Page
What is the general trend of ionization energy as you go down a group on the periodic table? 32. When an atom becomes an anion, what happens to its radius? 33. When an atom becomes a cation, what happens to its radius? 34. Where, generally, are the metals located on the periodic table? 35. Where, generally, are the nonmetals located on the ...
https://url.theworksheets.com/7rl310 Downloads
Preview and Download !


Periodic Table Worksheet #1 A. Write the electron configurations (all 3 ...
B. Periodic Table Group Properties 1. Define valence electrons: 2. Label the group numbers, period numbers, alkali metals, alkaline earth metals, transition metals, inner transition metals, halogens, noble gases, s-block, p-block, d-block, f-block, main group elements, and number of valence electrons on the following periodic table.
https://url.theworksheets.com/5fl6113 Downloads
Preview and Download !


Workbook - New York Science Teacher
Worksheet 5 : Types of elements and Properties Topic 2: The Periodic Table Set A: Terms and Definitions Objective: By defining these words, you should become more familiar with Periodic Table related terms and their definitions Define, neatly and clearly, the following Periodic Table related terms. 1. Periodic Law 2. Group 4. Metal 5. Nonmetal
https://url.theworksheets.com/1ft5190 Downloads
Preview and Download !


U2-LM1B-WorkSheet - Periodic Table - University of Texas at Austin
U2-LM1B-WorkSheet - Periodic Table 1. The law that states that the properties of the elements are periodic functions of their atomic number is _____. 2. Elements in the periodic table are arranged in order of increasing _____. 3. In the periodic table, each horizontal row is referred to as _____ On the other
https://url.theworksheets.com/nwt94 Downloads
Preview and Download !


Periodic Table Worksheets 1 - drrossymathandscience
9. The table below provides certain information about the symbol, the electron configuration, the name of the chemical family and the period number of four elements in the periodic table. Using the above information and the periodic table, fill in the empty boxes in the table. 10. The following table gives the description of four chemical elements.
https://url.theworksheets.com/nwm106 Downloads
Preview and Download !


Periodic Table Worksheets 1 - drrossymathandscience
9. The table below provides certain information about the symbol, the electron configuration, the name of the chemical family and the period number of four elements in the periodic table. Using the above information and the periodic table, fill in the empty boxes in the table. 10. The following table gives the description of four chemical elements.
https://url.theworksheets.com/nww145 Downloads
Preview and Download !


U2-LM1B-WorkSheet - Periodic Table - University of Texas at Austin
U2-LM1B-WorkSheet - Periodic Table 1. The law that states that the properties of the elements are periodic functions of their atomic number is _____. 2. Elements in the periodic table are arranged in order of increasing _____. 3. In the periodic table, each horizontal row is referred to as _____ On the other
https://url.theworksheets.com/nwo89 Downloads
Preview and Download !


PowerPoint Worksheet - Tina's Science Class
2. Who made the periodic table and when was it created? A Russian chemist and inventor named Dmitri Mendeleev created the periodic table in 1869. 3. What ability did the periodic table have? The periodic table was designed to make room for and predict the existence of elements that had not yet been discovered. INFORMATION ON THE PERIODIC TABLE 4.
https://url.theworksheets.com/6f9a96 Downloads
Preview and Download !
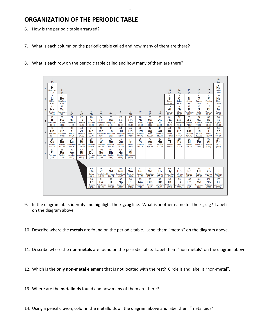

Periodic Table Worksheet - MARRIC
Periodic Table Worksheet Use a Periodic table to find the information asked for below: 1.What is the atomic number of: 2. What is the Atomic mass of: Calcium____ Calcium___ Iron _____ Iron_____ Gold_____ Uranium_____ Uranium_____ Copper_____ 3. How many protons do the following have? 4. How many electrons do the following have?
https://url.theworksheets.com/4hb4100 Downloads
Preview and Download !

Next results >>