Acids And Bases Worksheets Results
Intro to Acids & Bases Worksheet - Millersburg Area School District
When most people think of chemistry the first terms that come to mind are acids and bases. Many of the chemicals that people run across have some connection to acids and bases. For instance many people take medicine every day to cure heart burn. These medicines are (acids/bases). One of these comes in a blue bottle and is known as “Dr. MOM”.
https://url.theworksheets.com/3gh281 Downloads
Preview and Download !


4 Worksheet: Acids and bases - University of Pretoria
2.2 Write down the conjugated bases for the following acids: a. NH4 + b. HSO4 − c. H2O 2.3 Write down the conjugated acids of the following bases: a. H2PO4 − b. HSO4 − c. H2O 2.4 25 cm3 water is added tot 75 cm3 of a 0,13 mol·dm−3 sulphuric acid solution. Calculate the concentration of the diluted solution.
https://url.theworksheets.com/20os234 Downloads
Preview and Download !


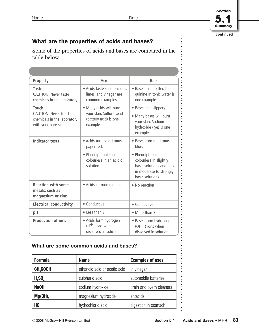
Section Name Date 5.1 Acids and Bases
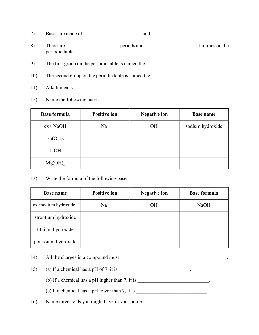
Acids versus bases 1. Compare and contrast acids and bases by completing the following table. Acids Bases definition pH what to look for in chemical formula production of ions electrical conductivity taste touch examples 2. Classify each of the following as an acid or a base. (a) + H 3 PO 4 (b) NH 4 OH (c) Mg(OH) 2 (d) has a pH of 4 (e) has a ...
https://url.theworksheets.com/1bxr132 Downloads
Preview and Download !

Acids, Bases & Salts
Scientists use a pH scale to measure the strength of acids and bases. The pH scale ranges from 0 to 14 . Substances with pH < 7 are acids ; the lower the pH, the stronger the acid. Substances with pH 7-14 are bases ; the higher the pH the stronger the base. Neutral solutions have a pH of exactly 7 . H O H ( ) O H (+) H
https://url.theworksheets.com/6byt115 Downloads
Preview and Download !

ACIDS AND BASES - newpathworksheets.com
ACIDS AND BASES. Most every liquid has acidic or basic traits. Acids and bases are two important compounds. Acids . An Acid is a type of sour substance. Examples of acids are lemon juice and vinegar . Remember: DO NOT TASTE SUBSTANCES during s cience e xperiments. Some Characteristics of A CIDS Important to Know : Â Acids taste sour
https://url.theworksheets.com/6bys102 Downloads
Preview and Download !

Naming Acids and Bases Worksheet Name: Chemistry
5) Name the following acids: Acid formula Positive ion Negative ion Acid name ex> HCl H+ Cl-hydrochloric acid HBr HNO 3 H 2 SO 4 H 3 PO 4 H 2 Se 6) Write the formula of the following acids: Acid name Positive ion Negative ion Acid formula ex> carbonic acid H+ CO 3 2-H 2 CO 3 hydrofluoric acid citric acid acetic acid hydroiodic acid
https://url.theworksheets.com/6byr112 Downloads
Preview and Download !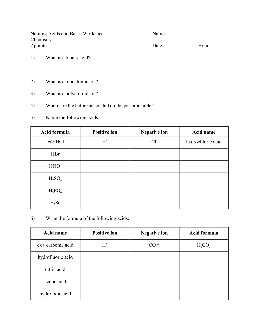

CHM 130 Acids, Bases, and Electrolytes Worksheet - gccaz.edu
2. Name 4 weak acids and write their formulas. What makes an acid weak? hydrofluoric acid HF, phosphoric acid H 3 PO 4, carbonic acid H 2 CO 3, acetic acid HC 2 H 3 O 2 Weak means very little ionized like 1-5%. Few H+ ions have come off the acid molecule in water. Few ions 3. Name 2 strong bases and write their formulas. What makes a base strong?
https://url.theworksheets.com/27b5226 Downloads
Preview and Download !


Acids, Bases and Salts - newpathworksheets.com
Acids, Bases and Salts Author: newpathworksheets.com Subject: Chemistry Keywords: Chemistry High School worksheets download, Chemistry High School study guides download, Acids, Bases and Salts, Chemistry worksheets Created Date: 9/1/2020 11:32:53 AM
https://url.theworksheets.com/27b2124 Downloads
Preview and Download !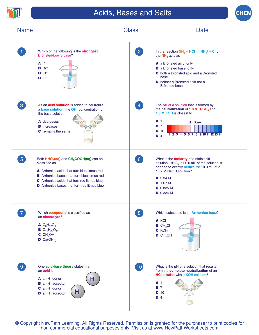
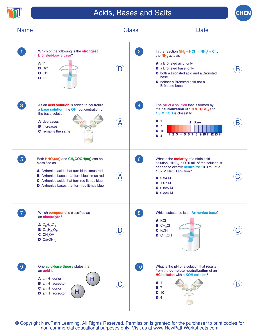

Unit 9: Acids, Bases, & Salts - Winston-Salem/Forsyth County Schools
7 Rules for Naming Acids: Binary Acids acids that START WITH H and are attached to a NONMETAL; _____ in acid’s formula 1st word in name Begin with _____-& follow it up immediately with the name of the other element while replacing the ending with -____ 2nd word in name add the word _____ as the second (last) word in the name
https://url.theworksheets.com/3y46107 Downloads
Preview and Download !


Acid and Base pH Calculations Supplemental Worksheet KEY
This reaction goes to completion because HCl and NaOH are strong acids and bases. So, all the NaOH disassociates into OH-and Na+ ions and all the HCl disassociates into Cl-and H3O+ ions. There is no limiting reactant because the HCl and NaOH are added in stoichiometric amounts. Calculate the pH Step 1: What is left in solution?
https://url.theworksheets.com/1bxg167 Downloads
Preview and Download !


Next results >>