Inorganic Chemistry Worksheets Results
Inorganic Chemistry with Doc M. Day 2. Slater Shielding and Effective ...
Inorganic Chemistry with Doc M. Day 2. Slater Shielding and Effective Nuclear Charge. Topics: 1. Shielding, Slater’s Rules, and effective nuclear charge, Zeff 2. Pertaining to cations and anions 5. Periodic trends revisited 3. Pertaining to main group elements 6. Ionic size (radius) 4. Pertaining to transition metal elements 1a.
https://url.theworksheets.com/3017137 Downloads
Preview and Download !

FORMULA WRITING AND NOMENCLATURE OF INORGANIC COMPOUNDS - chymist.com
Frequently, in chemistry, we come across a group of atoms that behave as if it were a single atom when it combines with another atom or group of atoms. Such a group of atoms is called a radical or polyatomic ion. For example, consider the following reaction between silver nitrate and ammonium chloride to form silver chloride and ammonium nitrate:
https://url.theworksheets.com/fbf170 Downloads
Preview and Download !

The Complete Organic Chemistry Worksheet - Alvin Independent School ...
14. Draw and name the five structural isomers of hexane (C 6H14) 15. Draw the structural formula for each of the following. a. 2-Methylpentane b. 2,2,4-Trimethylpentane, also called isooctane.This compound is the reference for octane ratings for gasoline.
https://url.theworksheets.com/1esf92 Downloads
Preview and Download !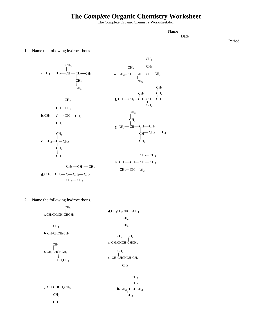

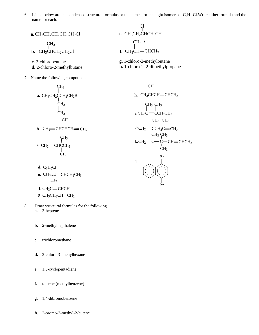
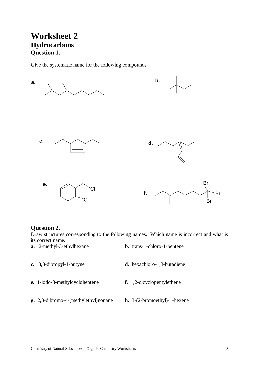
Worksheets for Organic Chemistry - van Maarseveen
Chemistry of Natural Substances – Organic Chemistry Worksheets 16 Question 6. Five organic liquids are subjected to a series of distinguishing tests to help determine their identity. The results are tabulated below. From the following list of possibilities, determine the identity of each liquid. Note that there may be more than one answer.
https://url.theworksheets.com/5rlz163 Downloads
Preview and Download !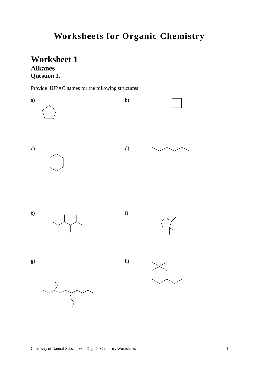

Inorganic Biochemistry - UNESCO
elements, generally considered “inorganic” are also essential for life. They are highlighted in the periodic table of Figure. 1. Their behavior within the biological context is the subject of Inorganic Biochemistry (or Bioinorganic Chemistry or Biological Inorganic Chemistry). Also relevant to the discipline are studies on model
https://url.theworksheets.com/6fo2111 Downloads
Preview and Download !


Chemistry 511 – Inorganic Chemistry - Department of Chemistry
Chemistry 511 – Inorganic Chemistry Fall 2004 KEEP THIS SYLLABUS FOR FUTURE REFERENCE Inorganic Chemistry: 3 credit hours Lecture: 11:00 – 11:50 am M,W,F, 2373 Chemistry * Lecturer: Nguyet T. Tran Phone Number: 265-0676 E–mail: nttran@chem.wisc.edu (please put Chem 511 in the subject heading)
https://url.theworksheets.com/6fo3108 Downloads
Preview and Download !

Mr. Kent’s Organic Chemistry Unit Notes I Basic Concepts A.
inorganic reactions IV Some Organic Compounds form _____. 1. Compounds with the same_____ but different ... OH- is hydroxide, but in organic chemistry side chains end with “-yl”. R-OH . Types of Alcohols Monohydroxy Alcohols- contains 1 –OH group Primary(1°)- the C-OH is attached to one other carbon (on the end)
https://url.theworksheets.com/67u0124 Downloads
Preview and Download !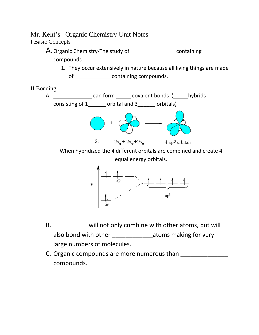


Electron Configuration Worksheet - Everett Community College
Write the unabbreviated electron configurations of the following elements: 1) copper 1s22s22p63s23p64s23d9 2) iodine 1s22s22p63s23p64s23d104p65s24d105p5 3) potassium ...
https://url.theworksheets.com/2lla127 Downloads
Preview and Download !


<< Previous results Next results >>