Balancing Equations Worksheets Results
Balancing Chemical Equations
KEY Chemistry: Balancing Chemical Equations Directions: First, balance each of the chemical equations below. Then, classify each reaction as synthesis, decomposition, single-replacement, or double-replacement.To earn full credit, write the words out
https://url.theworksheets.com/19sc210 Downloads
Preview and Download !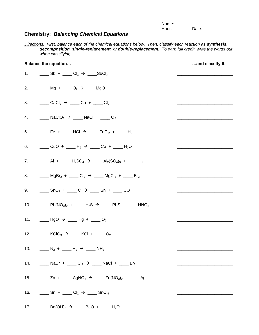


Chapter 7 Worksheet #1 Balancing Chemical Equations
Balancing Chemical Equations – Answer Key Balance the equations below: 1) 1 N 2 + 3 H 2 Æ 2 NH 3 2) 2 KClO 3 Æ 2 KCl + 3 O 2 3) 2 NaCl + 1 F 2 Æ 2 NaF + 1 Cl 2 4) 2 H 2 + 1 O 2 Æ 2 H 2O 5) 1 Pb(OH) 2 + 2 HCl Æ 2 H 2O + 1 PbCl 2 6) 2 AlBr 3 + 3 K 2SO 4 Æ 6 KBr + 1 Al 2(SO 4) 3 7) 1 CH 4 + 2 O 2 Æ 1 CO 2 + 2 H 2O 8) 1 C 3H 8 + 5 O 2 Æ 3 CO 2 + 4 H 2O 9) 2 C 8H 18 + 25 O 2 Æ 16 CO 2 ...
https://url.theworksheets.com/4pra176 Downloads
Preview and Download !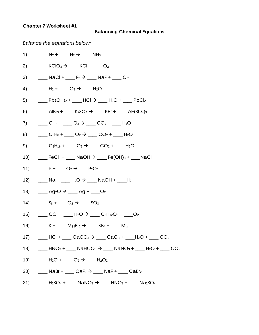


Balancing Equations - Weebly
Worksheet: Balancing Equations Name KEY 11. Fill in the blanks with the most appropriate term: chemical Reactants products equation tells the story of a chemical reaction. while are the starting substances in the reaction are the new substances that are formed. The
https://url.theworksheets.com/bx7216 Downloads
Preview and Download !


Worksheet: Balancing Equations Name I. Fill in the blanks with the most ...
When balancing equations, the only numbers that can be changed are _____; remember that _____ must never be changed in order to balance an equation. II. Balance the following equations: 1. Al + O 2 Al 2O 3 2. C 3H 8 + O 2 CO 2 + H 2O 3. Al(NO 3) 3 + NaOH Al(OH) 3 ...
https://url.theworksheets.com/1ckv136 Downloads
Preview and Download !


Balancing Equations Worksheet - mychemistryclass.net
Balancing Equations Worksheet – Answers Note to students: It is acceptable to leave spaces blank when balancing equations – blank spaces are interpreted as containing the number “1”. 1) 1 Na 3 PO 4 + 3 KOH 3 NaOH + 1 K 3 PO 4 2) 1 MgF 2 + 1 Li 2 CO 3 1 MgCO 3 + 2 LiF 3) 1 P 4 + 3 O 2 2 P 2 O 3 4) 2 RbNO 3 + 1 BeF 2 1 Be(NO 3) 2 + 2 RbF ...
https://url.theworksheets.com/4o1y132 Downloads
Preview and Download !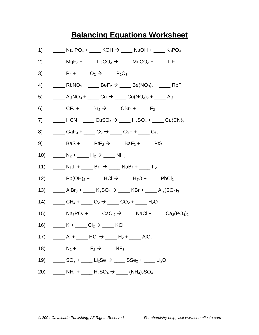


Balancing Equations Worksheet KEY
Balancing Equations Worksheet KEY 1. Zn (s) + 2 AgNO 3 (aq) ⇒ Zn(NO 3) 2 (aq) + 2 Ag (s) 2. N 2 (g) + 3 H 2 (g) ⇒ 2 NH 3 (g) 3. NaCl (aq) + AgC 2H 3O 2 (aq) ⇒ NaC 2H 3O 2 (aq) + AgCl (s) 4. 3 Mg(OH) 2 (aq) + 2 H 3PO 4 (aq) ⇒ 6 H 2O (l) + Mg 3(PO 4 ) 2 (aq) 5. 2 HNO 3 (aq) + Ni (s) ⇒ Ni(NO 3) 2 (aq) + H 2 (g) 6. Ba(HCO 3) 2 (s) ⇒ BaCO 3 (s) + H 2O (g) + CO
https://url.theworksheets.com/3ov174 Downloads
Preview and Download !


More Practice Balancing Redox - Livingston Public Schools
Finish balancing the equation by adding 1-120. Check to see that each element is balanced and that the charge is balanced. H20 Balance the following equations: Underline the oxidizing agent. 1--12S + H2S04 + H2S04 + N20 + Fe203 + NH3 HN03 HBr HI KN03 02 NO Br2 + 12 + NH3 100 H20 H2S + H20 N2 NO H20 H20
https://url.theworksheets.com/36bv105 Downloads
Preview and Download !


Worksheet # C31: Balancing Polyatomic Ionic Equations
Worksheet # C31: Balancing Polyatomic Ionic Equations Balance the following formula equations. Remember to count the polyatomic ions as units! Make sure you can name all of the compounds in # 1- 11. 1. ___ Na 3
https://url.theworksheets.com/4prc120 Downloads
Preview and Download !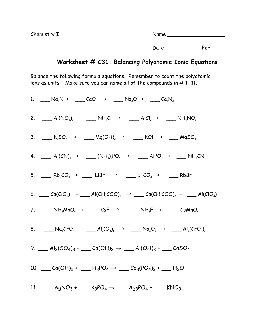


Name: Date: Balancing Equations
Name: Date: Balancing Equations About Chemistry http://chemistry.about.com Balance the following chemical equations. 1.
https://url.theworksheets.com/1ury118 Downloads
Preview and Download !


<< Previous results Next results >>