Acids And Bases Worksheets Results
Naming Acids and Bases Worksheet Name: Chemistry - Bruss's Page
5) Name the following acids: Acid formula Positive ion Negative ion Acid name ex> HCl H+ Cl-hydrochloric acid HBr HNO 3 H 2 SO 4 H 3 PO 4 H 2 Se 6) Write the formula of the following acids: Acid name Positive ion Negative ion Acid formula ex> carbonic acid H+ CO 3 2-H 2 CO 3 hydrofluoric acid citric acid acetic acid hydroiodic acid
https://url.theworksheets.com/2whx147 Downloads
Preview and Download !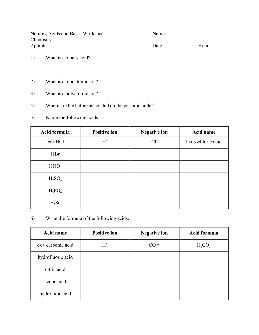
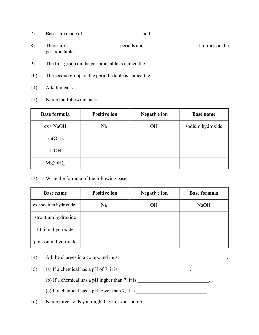

Name: Honors Chemistry Worksheet: Acids and Bases
8. Write a neutralization reaction for citric acid and potassium hydroxide. a.) What volume of 0.150 M citric acid is needed to react with 758.2 ml of 0.265 M potassium hydroxide? b.) If 46.20 ml of 0.232 M potassium hydroxide was required to neutralize 100.0 ml of citric acid what is the molarity of the citric acid?
https://url.theworksheets.com/3pgr68 Downloads
Preview and Download !


Worksheet 12 Acids & Bases: Weak Acids, Weak Bases, Ka b Objectives ’s ...
Ka’s are for acids and Kb’s are for bases. The relationship between Ka and Kb for a weak acid and it’s comjugate base is given by: For any weak acid, shown as HA, the percent dissociation is given by: 1.) Calculate the pH of the following solutions: 0.512 M HClO4 and 0.512 M HF (Ka = 6.76 x 10−4) pH HClO4 _____ pH HF_____ Page 1 of 6
https://url.theworksheets.com/4o1957 Downloads
Preview and Download !


Acids, Bases and Salts - newpathworksheets.com
Acids, Bases and Salts Author: newpathworksheets.com Subject: Chemistry Keywords: Chemistry High School worksheets download, Chemistry High School study guides download, Acids, Bases and Salts, Chemistry worksheets Created Date: 9/1/2020 11:32:54 AM
https://url.theworksheets.com/6byx58 Downloads
Preview and Download !

Give formulas for Conjugate acids and Bases - austincc.edu
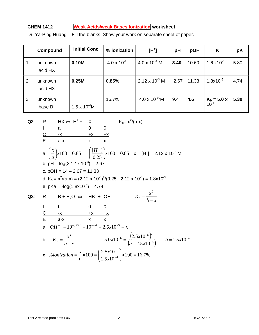
CHEM 1412 Weak Acids/weak Bases Ionization worksheet Dr. Ya-Ping Huang Fill the blanks. Show your work on separate sheet of paper. Compound Initial Conc. % ionization [H+] pH pOH K pK 1 unknown acid HX 0.10M 4.0 x 10-1 4.0 x 10-4 M 3.40 10.60 1.6 x10-6 5.80 2 unknown acid HX 0.25M 0.85% 2.12 x 10-3 M 2.67 11.33 1.8x10-5 4.74 3 unknown base B
https://url.theworksheets.com/6fg348 Downloads
Preview and Download !
CBSE Class 10 Science Acids Bases Salts Worksheet
(b) Since this milk is slightly basic than usual milk, acids produced to set the curd are neutralized by the base. Therefore, it takes a longer time for the curd to set. 13. Plaster. of. Paris. should. be. stored. in. a. moisture-proof. container. Explain. why? Answer-2. 2. of. and
https://url.theworksheets.com/6fg460 Downloads
Preview and Download !


<< Previous results